Your cart is currently empty!
Author: Dale Maxwell
Example Daily Activity Checklist and Timeline
[table id=14 /]
Pristiq Antidepressant Drug
5 WARNINGS AND PRECAUTIONS
5.1 Suicidal Thoughts and Behaviors in Children, Adolescents and Young Adults
Patients with major depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs. Suicide is a known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There has been a long-standing concern, however, that antidepressants may have a role in inducing worsening of depression and the emergence of suicidality in certain patients during the early phases of treatment. Pooled analyses of short-term placebo-controlled studies of antidepressant drugs (SSRIs and others) showed that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (ages 18 to 24) with major depressive disorder (MDD) and other psychiatric disorders. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction with antidepressants compared to placebo in adults aged 65 and older.
The pooled analyses of placebo-controlled studies in children and adolescents with MDD, obsessive compulsive disorder (OCD), or other psychiatric disorders included a total of 24 short-term studies of 9 antidepressant drugs in over 4,400 patients. The pooled analyses of placebo-controlled studies in adults with MDD or other psychiatric disorders included a total of 295 short-term studies (median duration of 2 months) of 11 antidepressant drugs in over 77,000 patients. There was considerable variation in risk of suicidality among drugs, but a tendency toward an increase in the younger patients for almost all drugs studied. There were differences in absolute risk of suicidality across the different indications, with the highest incidence in MDD. The risk differences (drug vs. placebo), however, were relatively stable within age strata and across indications. These risk differences (drug-placebo difference in the number of cases of suicidality per 1,000 patients treated) are provided in Table 1.
Table 1 Age Range Drug-Placebo Difference in Number of Cases of Suicidality per 1,000 Patients Treated Increases Compared to Placebo <18 14 additional cases 18 to 24 5 additional cases Decreases Compared to Placebo 25 to 64 1 fewer case ?65 6 fewer cases No suicides occurred in any of the pediatric studies. There were suicides in the adult studies, but the number was not sufficient to reach any conclusion about drug effect on suicide.
It is unknown whether the suicidality risk extends to longer-term use, i.e., beyond several months. However, there is substantial evidence from placebo-controlled maintenance studies in adults with depression that the use of antidepressants can delay the recurrence of depression.
All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, and mania, have been reported in adult and pediatric patients being treated with antidepressants for major depressive disorder as well as for other indications, both psychiatric and nonpsychiatric. Although a causal link between the emergence of such symptoms and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality.
Consideration should be given to changing the therapeutic regimen, including possibly discontinuing the medication, in patients whose depression is persistently worse, or who are experiencing emergent suicidality or symptoms that might be precursors to worsening depression or suicidality, especially if these symptoms are severe, abrupt in onset, or were not part of the patient’s presenting symptoms.
If the decision has been made to discontinue treatment, medication should be tapered, as rapidly as is feasible, but with recognition that abrupt discontinuation can be associated with certain symptoms [see Dosage and Administration (2.4) and Warnings and Precautions (5.7) for a description of the risks of discontinuation of PRISTIQ].
Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to healthcare providers. Such monitoring should include daily observation by families and caregivers.
Prescriptions for PRISTIQ should be written for the smallest quantity of tablets consistent with good patient management, in order to reduce the risk of overdose.
Screening patients for bipolar disorder
A major depressive episode may be the initial presentation of bipolar disorder. It is generally believed (though not established in controlled studies) that treating such an episode with an antidepressant alone may increase the likelihood of precipitation of a mixed/manic episode in patients at risk for bipolar disorder. Whether any of the symptoms described above represent such a conversion is unknown. However, prior to initiating treatment with an antidepressant, patients with depressive symptoms should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression. It should be noted that PRISTIQ is not approved for use in treating bipolar depression.
5.2 Serotonin Syndrome
The development of a potentially life-threatening serotonin syndrome has been reported with SNRIs and SSRIs, including PRISTIQ, alone but particularly with concomitant use of other serotonergic drugs (including triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, amphetamines, and St. John’s Wort), and with drugs that impair metabolism of serotonin (in particular, MAOIs, both those intended to treat psychiatric disorders and also others, such as linezolid and intravenous methylene blue).
Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination), seizures, and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). Patients should be monitored for the emergence of serotonin syndrome.
The concomitant use of PRISTIQ with MAOIs intended to treat psychiatric disorders is contraindicated. PRISTIQ should also not be started in a patient who is being treated with MAOIs such as linezolid or intravenous methylene blue. All reports with methylene blue that provided information on the route of administration involved intravenous administration in the dose range of 1 mg/kg to 8 mg/kg. No reports involved the administration of methylene blue by other routes (such as oral tablets or local tissue injection) or at lower doses. There may be circumstances when it is necessary to initiate treatment with a MAOI such as linezolid or intravenous methylene blue in a patient taking PRISTIQ. PRISTIQ should be discontinued before initiating treatment with the MAOI [see Contraindications (4.2) and Dosage and Administration (2.6)].
If concomitant use of PRISTIQ with other serotonergic drugs, including triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, buspirone, amphetamines, tryptophan, and St. John’s Wort is clinically warranted, patients should be made aware of a potential increased risk for serotonin syndrome, particularly during treatment initiation and dose increases.
Treatment with PRISTIQ and any concomitant serotonergic agents should be discontinued immediately if the above events occur and supportive symptomatic treatment should be initiated.
5.3 Elevated Blood Pressure
Patients receiving PRISTIQ should have regular monitoring of blood pressure since increases in blood pressure were observed in clinical studies [see Adverse Reactions (6.1)]. Pre-existing hypertension should be controlled before initiating treatment with PRISTIQ. Caution should be exercised in treating patients with pre-existing hypertension, cardiovascular, or cerebrovascular conditions that might be compromised by increases in blood pressure. Cases of elevated blood pressure requiring immediate treatment have been reported with PRISTIQ.
Sustained blood pressure increases could have adverse consequences. For patients who experience a sustained increase in blood pressure while receiving PRISTIQ, either dose reduction or discontinuation should be considered [see Adverse Reactions (6.1)].
5.4 Abnormal Bleeding
SSRIs and SNRIs, including PRISTIQ, may increase the risk of bleeding events. Concomitant use of aspirin, nonsteroidal anti-inflammatory drugs, warfarin, and other anticoagulants may add to this risk. Case reports and epidemiological studies (case-control and cohort design) have demonstrated an association between use of drugs that interfere with serotonin reuptake and the occurrence of gastrointestinal bleeding. Bleeding events related to SSRIs and SNRIs have ranged from ecchymosis, hematoma, epistaxis, and petechiae to life-threatening hemorrhages. Patients should be cautioned about the risk of bleeding associated with the concomitant use of PRISTIQ and NSAIDs, aspirin, or other drugs that affect coagulation or bleeding.
5.5 Angle Closure Glaucoma
Angle-Closure Glaucoma: The pupillary dilation that occurs following use of many antidepressant drugs including Pristiq may trigger an angle closure attack in a patient with anatomically narrow angles who does not have a patent iridectomy.
5.6 Activation of Mania/Hypomania
During all MDD phase 2 and phase 3 studies, mania was reported for approximately 0.02% of patients treated with PRISTIQ. Activation of mania/hypomania has also been reported in a small proportion of patients with major affective disorder who were treated with other marketed antidepressants. As with all antidepressants, PRISTIQ should be used cautiously in patients with a history or family history of mania or hypomania.
5.7 Discontinuation Syndrome
Discontinuation symptoms have been systematically and prospectively evaluated in patients treated with PRISTIQ during clinical studies in Major Depressive Disorder. Abrupt discontinuation or dose reduction has been associated with the appearance of new symptoms that include dizziness, nausea, headache, irritability, insomnia, diarrhea, anxiety, fatigue, abnormal dreams, and hyperhidrosis. In general, discontinuation events occurred more frequently with longer duration of therapy.
During marketing of SNRIs (Serotonin and Norepinephrine Reuptake Inhibitors), and SSRIs (Selective Serotonin Reuptake Inhibitors), there have been spontaneous reports of adverse events occurring upon discontinuation of these drugs, particularly when abrupt, including the following: dysphoric mood, irritability, agitation, dizziness, sensory disturbances (e.g., paresthesia, such as electric shock sensations), anxiety, confusion, headache, lethargy, emotional lability, insomnia, hypomania, tinnitus, and seizures. While these events are generally self-limiting, there have been reports of serious discontinuation symptoms.
Patients should be monitored for these symptoms when discontinuing treatment with PRISTIQ. A gradual reduction in the dose rather than abrupt cessation is recommended whenever possible. If intolerable symptoms occur following a decrease in the dose or upon discontinuation of treatment, then resuming the previously prescribed dose may be considered. Subsequently, the physician may continue decreasing the dose, but at a more gradual rate [see Dosage and Administration (2.4) and Adverse Reactions (6.1)].
5.8 Seizure
Cases of seizure have been reported in pre-marketing clinical studies with PRISTIQ. PRISTIQ has not been systematically evaluated in patients with a seizure disorder. Patients with a history of seizures were excluded from pre-marketing clinical studies. PRISTIQ should be prescribed with caution in patients with a seizure disorder.
5.9 Hyponatremia
Hyponatremia may occur as a result of treatment with SSRIs and SNRIs, including PRISTIQ. In many cases, this hyponatremia appears to be the result of the syndrome of inappropriate antidiuretic hormone secretion (SIADH). Cases with serum sodium lower than 110 mmol/L have been reported. Elderly patients may be at greater risk of developing hyponatremia with SSRIs and SNRIs. Also, patients taking diuretics or who are otherwise volume depleted can be at greater risk [see Use in Specific Populations (8.5) and Clinical Pharmacology (12.6)]. Discontinuation of PRISTIQ should be considered in patients with symptomatic hyponatremia and appropriate medical intervention should be instituted.
Signs and symptoms of hyponatremia include headache, difficulty concentrating, memory impairment, confusion, weakness, and unsteadiness, which can lead to falls. Signs and symptoms associated with more severe and/or acute cases have included hallucination, syncope, seizure, coma, respiratory arrest, and death.
5.10 Interstitial Lung Disease and Eosinophilic Pneumonia
Interstitial lung disease and eosinophilic pneumonia associated with venlafaxine (the parent drug of PRISTIQ) therapy have been rarely reported. The possibility of these adverse events should be considered in patients treated with PRISTIQ who present with progressive dyspnea, cough, or chest discomfort. Such patients should undergo a prompt medical evaluation, and discontinuation of PRISTIQ should be considered.
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the label.
- Hypersensitivity [see Contraindications (4)]
- Suicidal Thoughts and Behaviors in Adolescents and Young Adults [see Warnings and Precautions (5.1)]
- Serotonin Syndrome [see Warnings and Precautions (5.2)]
- Elevated Blood Pressure [see Warnings and Precautions (5.3)]
- Abnormal Bleeding [see Warnings and Precautions (5.4)]
- Angle Closure Glaucoma [see Warnings and Precautions (5.5)]
- Activation of Mania/Hypomania [see Warnings and Precautions (5.6)]
- Discontinuation Syndrome [see Warnings and Precautions (5.7)]
- Seizure [see Warnings and Precautions (5.8)]
- Hyponatremia [see Warnings and Precautions (5.9)]
- Interstitial Lung Disease and Eosinophilic Pneumonia [see Warnings and Precautions (5.10)]
6.1 Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in clinical practice.
Patient exposure
PRISTIQ was evaluated for safety in 8,394 patients diagnosed with major depressive disorder who participated in multiple-dose pre-marketing studies, representing 2,784 patient-years of exposure. Of the total 8,394 patients exposed to at least one dose of PRISTIQ; 2,116 were exposed to PRISTIQ for 6 months, representing 1,658 patient-years of exposure, and 421 were exposed for one year, representing 416 patient-years of exposure.
Adverse reactions reported as reasons for discontinuation of treatment
In the pre-marketing pooled 8-week placebo-controlled studies in patients with MDD, 1,834 patients were exposed to PRISTIQ (50 to 400 mg). Of the 1,834 patients, 12% discontinued treatment due to an adverse reaction, compared with 3% of the 1,116 placebo-treated patients. At the recommended dose of 50 mg, the discontinuation rate due to an adverse reaction for PRISTIQ (4.1%) was similar to the rate for placebo (3.8%). For the 100 mg dose of PRISTIQ the discontinuation rate due to an adverse reaction was 8.7%.
The most common adverse reactions leading to discontinuation in at least 2% and at a rate greater than placebo of the PRISTIQ treated patients in the short-term studies, up to 8 weeks, were: nausea (4%); dizziness, headache and vomiting (2% each). In a longer-term study, up to 9 months, the most common was vomiting (2%).
Common adverse reactions in placebo-controlled MDD studies
The most commonly observed adverse reactions in PRISTIQ treated MDD patients in pre-marketing pooled 8-week, placebo-controlled, fixed-dose studies (incidence ? 5% and at least twice the rate of placebo in the 50 or 100 mg dose groups) were: nausea, dizziness, insomnia, hyperhidrosis, constipation, somnolence, decreased appetite, anxiety, and specific male sexual function disorders.
Table 2 shows the incidence of common adverse reactions that occurred in ? 2% of PRISTIQ treated MDD patients and twice the rate of placebo at any dose in the pre-marketing pooled 8-week, placebo-controlled, fixed dose clinical studies
Table 2: Common Adverse Reactions (? 2% in any Fixed-Dose Group and Twice the Rate of Placebo) in Pre-marketing Pooled MDD 8-Week Placebo-Controlled Studies Percentage of Patients Reporting Reaction PRISTIQ System Organ Class
Preferred TermPlacebo
(n=636)50 mg
(n=317)100 mg
(n=424)200 mg
(n=307)400 mg
(n=317)Cardiac disorders Blood pressure increased 1 1 1 2 2 Gastrointestinal disorders Nausea 10 22 26 36 41 Dry mouth 9 11 17 21 25 Constipation 4 9 9 10 14 Vomiting 3 3 4 6 9 General disorders and administration site conditions Fatigue 4 7 7 10 11 Chills 1 1 <1 3 4 Feeling jittery 1 1 2 3 3 Metabolism and nutrition disorders Decreased appetite 2 5 8 10 10 Nervous system disorders Dizziness 5 13 10 15 16 Somnolence 4 4 9 12 12 Tremor 2 2 3 9 9 Disturbance in attention <1 <1 1 2 1 Psychiatric disorders Insomnia 6 9 12 14 15 Anxiety 2 3 5 4 4 Nervousness 1 <1 1 2 2 Abnormal dreams 1 2 3 2 4 Renal and urinary disorders Urinary hesitation 0 <1 1 2 2 Respiratory, thoracic and mediastinal disorders Yawning <1 1 1 4 3 Skin and subcutaneous tissue disorders Hyperhidrosis 4 10 11 18 21 Special Senses Vision blurred 1 3 4 4 4 Mydriasis <1 2 2 6 6 Vertigo 1 2 1 5 3 Tinnitus 1 2 1 1 2 Dysgeusia 1 1 1 1 2 Vascular disorders Hot flush <1 1 1 2 2 Sexual function adverse reactions
Table 3 shows the incidence of sexual function adverse reactions that occurred in ? 2% of PRISTIQ treated MDD patients in any fixed-dose group (pre-marketing pooled 8-week, placebo-controlled, fixed -dose, clinical studies).
Table 3: Sexual Function Adverse Reactions (? 2% in Men or Women in any PRISTIQ Group) During the On-Therapy Period PRISTIQ Placebo
(n=239)50 mg
(n=108)100 mg
(n=157)200 mg
(n=131)400 mg
(n=154)Men only Anorgasmia 0 0 3 5 8 Libido decreased 1 4 5 6 3 Orgasm abnormal 0 0 1 2 3 Ejaculation delayed <1 1 5 7 6 Erectile dysfunction 1 3 6 8 11 Ejaculation disorder 0 0 1 2 5 Ejaculation failure 0 1 0 2 2 Sexual dysfunction 0 1 0 0 2 PRISTIQ Placebo
(n=397)50 mg
(n=209)100 mg
(n=267)200 mg
(n=176)400 mg
(n=163)Women only Anorgasmia 0 1 1 0 3 Other adverse reactions observed in premarketing and postmarketing clinical studies
Other infrequent adverse reactions, not described elsewhere in the label, occurring at an incidence of < 2% in MDD patients treated with PRISTIQ were:
Cardiac disorders – Tachycardia.
General disorders and administration site conditions – Asthenia.
Investigations – Weight increased, liver function test abnormal, blood prolactin increased.
Musculoskeletal and connective tissue disorders – Musculoskeletal stiffness.
Nervous system disorders –Syncope, convulsion, dystonia.
Psychiatric disorders – Depersonalization, bruxism.
Renal and urinary disorders – Urinary retention.
Skin and subcutaneous tissue disorders – Rash, alopecia, photosensitivity reaction, angioedema.
In clinical studies, there were uncommon reports of ischemic cardiac adverse reactions, including myocardial ischemia, myocardial infarction, and coronary occlusion requiring revascularization; these patients had multiple underlying cardiac risk factors. More patients experienced these events during PRISTIQ treatment as compared to placebo.
Laboratory, ECG and vital sign changes observed in MDD clinical studies
The following changes were observed in pre-marketing placebo-controlled, short-term MDD studies with PRISTIQ.
Lipids
Elevations in fasting serum total cholesterol, LDL (low density lipoproteins) cholesterol, and triglycerides occurred in the controlled studies. Some of these abnormalities were considered potentially clinically significant.
The percentage of patients who exceeded a predetermined threshold value is shown in Table 4.
Table 4: Incidence (%) of Patients With Lipid Abnormalities of Potential Clinical Significance* PRISTIQ Placebo 50 mg 100 mg 200 mg 400 mg Total Cholesterol
*(Increase of ? 50 mg/dl and an absolute value of ? 261 mg/dl)2 3 4 4 10 LDL Cholesterol
*(Increase ? 50 mg/dl and an absolute value of ? 190 mg/dl)0 1 0 1 2 Triglycerides, fasting
*(Fasting: ? 327 mg/dl)3 2 1 4 6 Proteinuria
Proteinuria, greater than or equal to trace, was observed in the pre-marketing fixed-dose controlled studies (see Table 5). This proteinuria was not associated with increases in BUN or creatinine and was generally transient.
Table 5: Incidence (%) of Patients with Proteinuria in the Fixed-dose Clinical Studies PRISTIQ Placebo 50 mg 100 mg 200 mg 400 mg Proteinuria 4 6 8 5 7 Vital sign changes
Table 6 summarizes the changes that were observed in placebo-controlled, short-term, pre-marketing studies with PRISTIQ in patients with MDD (doses 50 to 400 mg).
Table 6: Mean Changes in Vital Signs at Final on Therapy for All Short-term, Fixed-dose Controlled Studies PRISTIQ Placebo 50 mg 100 mg 200 mg 400 mg Blood pressure Supine systolic bp (mm Hg) -1.4 1.2 2.0 2.5 2.1 Supine diastolic bp (mm Hg) -0.6 0.7 0.8 1.8 2.3 Pulse rate Supine pulse (bpm) -0.3 1.3 1.3 0.9 4.1 Weight (kg) 0.0 -0.4 -0.6 -0.9 -1.1 Treatment with PRISTIQ at all doses from 50 mg per day to 400 mg per day in controlled studies was associated with sustained hypertension, defined as treatment-emergent supine diastolic blood pressure (SDBP) ?90 mm Hg and ?10 mm Hg above baseline for 3 consecutive on-therapy visits (see Table 7). Analyses of patients in PRISTIQ pre-marketing short-term controlled studies who met criteria for sustained hypertension revealed a consistent increase in the proportion of patients who developed sustained hypertension. This was seen at all doses with a suggestion of a higher rate at 400 mg per day.
Table 7: Proportion of Patients with Sustained Elevation of Supine Diastolic Blood Pressure Treatment Group Proportion of Patients with Sustained Hypertension Placebo 0.5% PRISTIQ 50 mg per day 1.3% PRISTIQ 100 mg per day 0.7% PRISTIQ 200 mg per day 1.1% PRISTIQ 400 mg per day 2.3% Orthostatic hypotension
In the pre-marketing short-term, placebo-controlled clinical studies with doses of 50 to 400 mg, systolic orthostatic hypotension (decrease ?30 mm Hg from supine to standing position) occurred more frequently in patients ?65 years of age receiving PRISTIQ (8%, 7/87) versus placebo (2.5%, 1/40), compared to patients <65 years of age receiving PRISTIQ (0.9%, 18/1,937) versus placebo (0.7%, 8/1,218).
6.2 Postmarketing Experience
The following adverse reaction has been identified during post-approval use of PRISTIQ. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
Skin and subcutaneous tissue disorders – Stevens-Johnson syndrome.
Gastrointestinal disorders – Pancreatitis acute.
Link to the Full Document on Manufacturer Page
Long Term Effects Of Morphine On The Body
Using morphine can cause both reversible and permanent changes to the body. In fact, morphine affects almost all systems and organs in the human body, and excessive or prolonged use can bring dangerous consequences.
However, the greatest risk of using morphine is death resulting from the gradual shutdown of the respiratory system.
What are some of the benign or even the more serious effects of morphine on the body? We examine here.
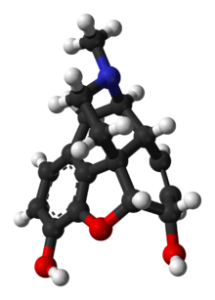
Primary effects of morphine on the body
- abnormal thoughts
- alterations in personality
- analgesia (inability to feel pain)
- constipation
- cramping
- dehydration
- delirium
- disconnectedness
- disturbed sleeping
- drowsiness
- headaches
- nausea
- the possibility of seizures
- reduced gastrointestinal motility
- sedation
- severe depression
- slowed reaction time
- trouble walking
- vomiting
How morphine damages the cardiovascular system
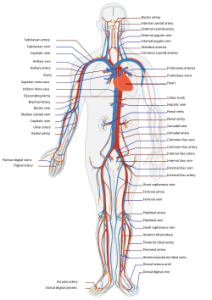
- chest pain
- collapsed veins
- hypotension
- low blood pressure
- vasodilation
How morphine damages the heart
- depressed heart rate
- endocarditis (inflammation of the inner lining of the heart)
- fast, pounding, or irregular heartbeat or pulse
How morphine damages the mouth
- dry mouth
- swelling of lips and tongue
How morphine damages the kidneys
- difficulties passing urine
- painful urination
- renal damage
How morphine damages the lungs
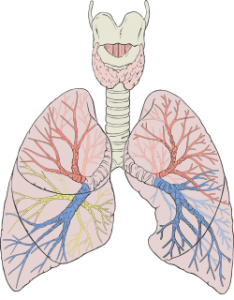
- respiratory acidosis
- respiratory depression
- shallow breathing
How morphine damages the skin
- flushing of face and neck due to dilatation of subcutaneous blood vessels
- itching
- rashes
How morphine damages the liver
- hepatic damage
- hepatitis
- increases in hepatic enzymes
How morphine damages the ear, nose and throat
- pounding in the ears
- trouble swallowing
How morphine damages the eyes
- change in the ability to see colors, especially blue or yellow
- pupils fixed and constricted
- red eyes
- swelling of the eyelids or around the eyes
- vision problems
Body Dependence And Tolerance To Morphine
Finally, it is important to note that using morphine for longer than a week or two can lead to dependence. When you become dependent on morphine, you experience withdrawal symptoms when you lower or cease dosing. With use over time, morphine also causes tolerance…meaning that more of the drug is needed in order to be effective.
I recommend managing pain with coffee enemas.
Image credit: wikipedia.org
Distilled Water The Shocking Truth
[svpVideo v=1]
side effects of XTANDI (enzalutamide)
Highlights of side effects are listed here link to Manufacturer found at bottom of post – I highly recommend you study each and every detail, use google and youtube to find stories that are not included in the official report.
What are the possible side effects of XTANDI?
XTANDI may cause serious side effects including:
Seizure
If you take XTANDI you may be at risk of having a seizure. Avoid activities where losing consciousness could seriously harm you or someone else. Tell your doctor right away if you lose consciousness or have a seizure. Your doctor will stop XTANDI if you have a seizure during treatment.
Posterior Reversible Encephalopathy Syndrome (PRES)
If you take XTANDI you may be at risk of developing a condition involving the brain called PRES. Tell your healthcare provider right away if you have a seizure or quickly worsening symptoms such as headache, decreased alertness, confusion, reduced eyesight, blurred vision or other visual problems. Your healthcare provider will do a test to check for PRES. Your healthcare provider will stop XTANDI if you develop PRES.
The most common side effects of XTANDI include:
- weakness or feeling more tired than usual
- back pain
- decreased appetite
- constipation
- joint pain
- diarrhea
- hot flashes
- upper respiratory tract infection
- swelling in your hands, arms, legs, or feet
- shortness of breath
- muscle and bone pain
- weight loss
- headache
- high blood pressure
- dizziness
- vertigo (a feeling that you or things around you are moving or spinning)
XTANDI may cause infections, falls and injuries from falls. Tell your doctor if you have signs or symptoms of an infection or if you fall.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of XTANDI. For more information, ask your healthcare provider or pharmacist.
Download the official PDF http://www.astellas.us/docs/us/12A005-ENZ-WPI.pdf
Root Canal Removal Resources
Eliminate the root canals and make sure the socket is scraped by 1mm to remove the tooth ligament. If any remains you could develop cavitations (pockets where bacteria can make a home).
Contact https://www.hugginsappliedhealing.com/ for a referral to a properly trained dentist.
Well recognized contributors to disease include:
Root canals. Any root canals you have in your body are releasing toxins. A root canal tooth is dead and no one would leave a dead part in a body. It makes no sense that dentists do this.
Youtube Videos
https://www.youtube.com/results?search_query=Root+canal+hazards
Price-Pottenger foundation lists over 20 books, courses and reports documenting the root canal coverup.http://ppnf.org/?s=root+canals&post_type=0
[svpVideo v=1]
And More on Root Canals
[svpVideo v=2]
Book Mentioned in video
After Dental Surgery
Re-inoculate your gut bacteria
https://www.drkelleyenzymes.com/product/liviotic-350-clinical-strength-high-potency-multispecies-multistrain-probiotic/
Non-cancer Immune and digestion challange
MV asks Dale (in completed questionnaire):
Aches, Pains, and Concerns:
Pain Frequency and duration intestinal? Pain (low back), jaw pain, leg pain, arm pain, swollen fingers, stiff hands, Hashimoto’s, neck pain, swollen throat, itchy throat, burning face and eyes, congested nose, watery eyes, burning tongue, constipation, low elastase, internal tremors as a reaction to certain food, ear eczema, fluid from the ears, cheek pressure, nausea from heavy foods, abdominal pain….
List other Concerns REACTING TO JUST ABOUT ANY FOOD I HAVE, PRIMARY IMMUNE DEFICIENCY
Dale Responds:
[svpVideo v=1]
Suggested
21-day detox with Clear Multivite
https://www.drkelleyenzymes.com/product/clear-multivite-798-meal-replacement/
Liver Support
Liver Support: Initial two months at 4 Caps AM and 4 Caps PM then reduce to 2 AM and 2 PM. If you notice any return of discomfort, go back to the higher dose.
https://www.drkelleyenzymes.com/product/liverite-liver-detox/
Re-introduce Gut Bacteria
For you, I suggest two Bottles are needed.
https://www.drkelleyenzymes.com/product/liviotic-350-clinical-strength-high-potency-multispecies-multistrain-probiotic/
Remove the Root Canals
This is likely either the cause or a contribution to your autoimmune problem. Do not believe me blindly, here is the science:
Diet Plant:
Why Diet 95 % whole food plant based 5% animal – no free flowing oils
Increase your body’s ability to absorb nutrition with six months of Okra Pepsin
Standard Process Okra Pepsin E3, 150 Capsules (50 day supply)
Low Elastase
You may want to test consuming Solozyme away from food to assist in healing your pancreas and if you can afford to uses before meals will help better than the 325 normally used with food This is due to the fact that the USP enzyme dows not have the trypsin that is needed for elastase to be activated.
https://www.drkelleyenzymes.com/product/dr-kelleys-pancreatic-enzyme-solozyme-360-capsules-750-mg/
Support digestion with some Pancreatin
Pancreatic Enzyme Capsules or Tablets? What is Best For You?
How to Figure out how much Pancreatin:
Pancreatin, 1200 mg, 1,000 Capsules, Take 30 Minutes Before Food
Are grapes, dates, raisins and dry figs good to eat?
PB Asks: Hello Dale, Are grapes, dates, raisins and dry figs good to eat?
Dale Responds:
- Dried fruits may have preservatives added, which should be avoided.
- Much more calories per ounce.
- Best to eat the fruit (organic) that is fresh when you can.
Dr. Gregor comments on dried fruits:
[svpVideo v=1]
and
Which Fruit Fights Cancer Better?
[svpVideo v=2]
What To Do If You Get Food Poisoning Symptoms
You may get food poisoning from a number of locations and circumstances. I suggest it is best to be prepared wth a clinical strength probiotic.
Food poisoning is a common infectious condition that affects millions of people in the United States each year.
Most commonly, people complain of vomiting, diarrhea, and cramping abdominal pain.
Standard treatment for food poisoning focuses on keeping the affected person well hydrated.
Most cases of food poisoning resolve on their own, however, re-inoculation will speed recovery and boost your immune system.
Other circumstances that can damage your microbiome (gut bacteria) you should be prepared for include:
Dental Visit – I suggest you refuse any fluoride or bleaching and understand that if you choose to take an antibiotic, you will kill as much as 33% of the healthy gut bacteria.
Travel – you may get bacteria that the locals are fine with but give you big problems.
Travel by airline and cruise ships have been reported in the news for some time now, and it is unlikely to magically disappear from being an occasional problem. It is a big enough problem that the World Health Organization is now in its third revision of the guidelines.

I suggest that you take one packet at the first sign of distress and a total of two times per day for three days should repopulate your gut.
Use common sense and seek a health practitioner if symptoms to not quickly resolve. You should seek medical care if they have an associated fever, blood in their stool (rectal bleeding), signs and symptoms of dehydration, or if their symptoms do not resolve after a couple of days
The one we recommend you have on hand keeps well and travels well. Each serving is listed here:
https://www.drkelleyenzymes.com/product/liviotic-350-clinical-strength-high-potency-multispecies-multistrain-probiotic/
Ship Image from Wikipedia
Distilled Water Secrets UN Doesn’t Want You to Know!
[svpVideo v=1]
I have to agree with this video, we documented in this book.
Get the Book “Dirty Water Warning” by Dale Maxwell (c) 2008 as part of the collection
38 year cancer survivor
How I survived terminal cancer w/ alternative cancer treatments
Rick Hill reports how he got well
[svpVideo v=1]
Detoxing Coffee Enemas
Juicing
Supplements 25-38 per day for 38 years
no gluten Comment from Dale, I do not recommend any packaged foods (even gluten free.)
Attitude 23 minutes
- Is your life worth Saving?
- Is the life you left behind worth going back to?
- What needs to change for question 1 and 2 to be answered YES!
- Are you “All-In?”
Waterless/greaseless cookware recommended.

